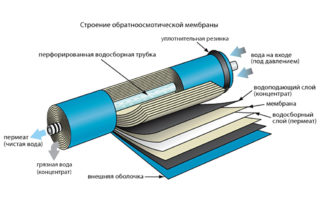
A reverse osmosis filter purifies water by passing it under pressure through a low-permeability membrane. Purification is used in domestic and industrial conditions to return water qualities suitable for drinking and use in household needs. After setting up new equipment, filtration is in the current operating mode, but over time, the elements become clogged with debris and the performance of the system is noticeably reduced. To restore the level of osmosis power, the membranes are cleaned.
Signs of membrane contamination
To return the performance of the osmotic filter to the initial level, it is recommended to flush the membranes with reagents containing acids and alkalis in order to remove accumulated dirt. With the help of professional compounds, sludge and organic accumulations formed during the operation of the system are removed from the surface of the filters.
The membrane in the reverse osmosis filter is located in the body of the purifier. Quantitative capacity can be from one to seven pieces. They are structurally distinguished:
- wound in a spiral shape,
- fiber-based.
The most popular are spiral type elements. By the type of assembly, they are a pair of membranes wound on a central branch pipe. With constant operation, after a certain time, a decrease in productivity becomes noticeable, a loss of the required qualitative composition of purified water, or a large pressure drop across individual membrane elements. All these indicators indicate blockages.
The types of deposits on filtration elements differ in their physicochemical properties and the method of formation. The most common are:
- Cretaceous deposits. The main sign of calcium carbonate contamination is a light coating on the edges of the osmosis membranes. It can be yellow or beige, rarely white. This type of plaque is neutralized by hydrochloric acid and is accompanied by a slight bubbling. When the sludge contains only calcium carbonate, the sediment disappears completely and the solvent does not change color. The color transition and the appearance of extraneous fractions indicate that other substances were also contained in the plaque.
- Gypsum deposits. Calcium sulphate deposits are most commonly caused by filtration of sea and underground brackish water. After the formation of the first crystals on the membrane against the background of constant replenishment of foreign substances, a chain reaction occurs, pollution cannot be stopped. Symptoms by which a calcium sulfate deposit is recognized is a layer of light color, as in the case of chalk deposits. The difference is that the clogging of the filter elements occurs much faster, and the substances cannot be dissolved with hydrochloric acid.
- Iron oxide. A brown plaque remains on the membrane, the origin of which is still not reliably clear. Contamination is thought to be due to some bacteria leaving iron hydroxide particles on the membranes.
- Silicon deposits. In the process of polymerization, insoluble silica gel is formed, which enters into a chemical reaction with iron, calcium and other substances. The growth rate of plaque increases with the influx of contaminated water. Stubborn plaque cannot be removed.
- Biological debris.Black plaque is caused by mold, mildew or sludge accumulation. Biological contaminants often accumulate in the form of mucus and a film on the membrane or housing of the filtration system. Raids of this kind are dangerous because, destroying the elements, they can get into the drinking water and cause various diseases.
Biological plaque adheres to filters due to its physical properties: roughness, hydrophobicity and surface charge. After stopping, the bacteria begin to secrete polysaccharides, which leads to increased colony growth and increased pollution.
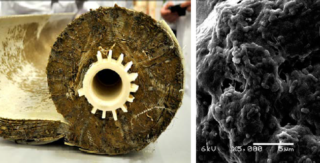
To prevent the appearance of biological contaminants in reverse osmosis, it is necessary to strictly monitor the cleanliness of the pre-filtration systems. The likelihood of bacterial growth increases during downtime. In industrial installations, with a delay in water purification for a day, all membranes involved in production are inseminated. To remove the infection, it is necessary to carry out a set of disinfecting measures using chemicals.
The symptoms for all types of contamination are:
- general decrease in the performance of the reverse osmosis system up to 20%;
- deterioration of the qualitative composition of pure water;
- significant, up to 20%, pressure difference between polluted water and permeate.
To restore the power of the system, it is recommended to clean the reverse osmosis membrane using chemicals.
Effective Membrane Flushing Methods

When using reverse osmosis filtration, the health of the consumer depends on the quality and purity of the membrane. There is mechanical and chemical flushing.
Mechanical is carried out by changing the water pressure in the opposite direction, which leads to the expulsion and removal of plaque. In industrial filters, such manipulations are carried out up to five times per hour for up to 30 seconds. The result achieved by machining is influenced by the flow rate of the volume of incoming water. The higher it is, the better the cleaning.
Before carrying out chemical flushing, it is necessary to establish the type of pollutant. Often, the situation is aggravated by the presence of different types of plaque, which leads to the use of cleaning in several stages using solutions of different acidity.
To purify osmosis used at home, you must:
- turn off the tap on the storage tank;
- close the valve in front of the filter and unscrew the pressure relief valve;
- disconnect the JG tube and the inlet fitting, remove the filter;
- flush the reverse osmosis membrane with citric acid at the rate of 150 grams of acid per 1 liter of water, stand for 12 hours and place in the system, performing the actions in the reverse order.
Membrane cleaning in industrial systems consists of chemical rinsing and disinfection treatments. The substances used must be safe for filters, therefore, the required concentration and duration of the procedure must be determined in advance.
For systems with low productivity, the pressure change method is used as a regeneration. The valve is unscrewed at the concentrate area, which leads to its discharge in significant volumes and the removal of a large percentage of contaminating deposits. It is not always possible to use this method on powerful installations. To carry out high-quality cleaning, you must:
- analyze the composition of the incoming water;
- monitor the condition of the equipment;
- select the required type of flushing solution and its concentration;
- set the frequency and duration of flushing;
- remove residual solution from the membrane by flushing.
The use of a reverse osmosis filter presupposes strict adherence to operating conditions. Therefore, cleaning the membranes is necessary to ensure the smooth functioning of the system and to prevent the appearance of contamination.